31 | Add to Reading ListSource URL: www.fda.govLanguage: English |
|---|
32 | Add to Reading ListSource URL: www.fda.govLanguage: English |
|---|
33 | Add to Reading ListSource URL: www.fda.govLanguage: English |
|---|
34 | Add to Reading ListSource URL: mrh.eac.intLanguage: English - Date: 2014-01-25 14:41:08
|
|---|
35 | Add to Reading ListSource URL: www.fda.govLanguage: English |
|---|
36 | Add to Reading ListSource URL: mrh.eac.intLanguage: English - Date: 2014-01-25 14:40:18
|
|---|
37 | Add to Reading ListSource URL: www.fda.govLanguage: English - Date: 2002-07-10 10:01:47
|
|---|
38 | Add to Reading ListSource URL: idl-bnc.idrc.caLanguage: English - Date: 2011-09-02 02:05:49
|
|---|
39 | Add to Reading ListSource URL: www.fda.govLanguage: English |
|---|
40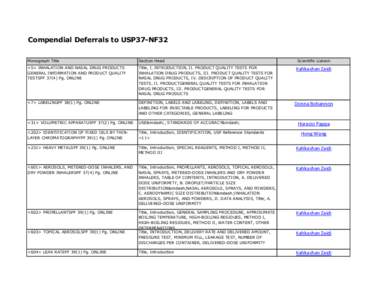 | Add to Reading ListSource URL: www.usp.orgLanguage: English - Date: 2015-01-07 12:29:13
|
|---|